|
|
|
|
|
ANTIMICROBIAL AND ANTIOXIDANT ACTIVITIES OF DATE PALM FRUIT EXTRACTS (Phoenix dactylifera L)
Olawale-Success, Olajumoke
Oluwagbemisola 1![]()
![]()
1 Department
of Biological Sciences, Faculty of Computing and Applied Sciences, Dominion
University, Km 24, Ibadan-Lagos Express Way, Ibadan, Nigeria
|
|
ABSTRACT |
||
|
Phoenix
dactylifera L., also known as date or date palm, a
plant flower in the palm family, Arecaceae, cultivated for its
edible pleasant fruit. The proximate outcome showed that the date
palm fruit has high carbohydrate content (84.6%), followed by crude fibre
(8.66%), while the fat content was the lowest (0.21%). The date palm fruit contains
high β
-Carotene (87.91µg) and lower lycopene (0.006µg). The antimicrobial
susceptibility of the test organisms, Staphylococcus aureus, Escherichia coli, Salmonella typhi, Bacillus
cereus and Aspergillus
niger to extracts
of date palm fruit revealed that all the microorganisms were susceptible to
the extracts. |
|||
|
Received 09 July 2024 Accepted 12 August 2024 Published 22 August 2024 Corresponding Author Olawale-Success,
Olajumoke Oluwagbemisola, o.olawale-success@dominionuniversity.edu.ng
DOI 10.29121/ShodhAI.v1.i1.2024.5 Funding: This research
received no specific grant from any funding agency in the public, commercial,
or not-for-profit sectors. Copyright: © 2024 The
Author(s). This work is licensed under a Creative Commons
Attribution 4.0 International License. With the
license CC-BY, authors retain the copyright, allowing anyone to download,
reuse, re-print, modify, distribute, and/or copy their contribution. The work
must be properly attributed to its author.
|
|||
|
Keywords: Phoenix Dactylifera L. (Date Palm), Antioxidant,
Antimicrobial Susceptibility, Staphylococcus Aureus, Escherichia Coli, Salmonella
Typhi, Bacillus Cereus and Aspergillus Niger |
|||
1. INTRODUCTION
Phoenix dactylifera L., also known as date or date palm, is a plant flower in the palm family, Arecaceae, cultivated for its edible sweet fruit. P. dactylifera is the type species of genus Phoenix, which contains 12–19 species of wild date palms, and is the major source of commercial production Rahimi (2015). Date is such a staple food from the Middle East and the Indus Valley, being existing for thousands of years. Archaeological evidence of date cultivation in Arabia from the 6th millennium BC was examined by availability. Patel et al. (2012).
Anmnual global production of dates amounts to a total of
8.5 million metric tons,
nations from the Middle East and North Africa seem the largest producers of it.
The plant species of dactylifera "date-bearing" emanate from
the Greek ter daktylos,
which connotes "date" (also
"finger") and fero, meaning "I bear". The fruit
is known as a date. The fruit's English name (through Old French), as well as
the Latin all came from the Greek word for "finger", dáktulos,
due to elongated shape of the fruit. Fossil revealed how the date palm has been in existence
for at least many years Upadhyay
et al. (2014). Dates have always
included in staple food of
the Middle East and the Indus valley for thousands of years. The
ancient Hebrew turn the fruit into wine, vinegar,
bread, and cakes, fruit stones to is also used to fatten livestock and the wood
for making utensils. They are very high in some essential nutrients and have a
variety of advantages and usage. Dates have an excellent nutrition profile
since they are dried, their calorie content is higher than most fresh fruit Grover
& Patni (2013). Dates usually yields
various antioxidants that have a number of health benefits to offer, including
a reduced risk of several diseases. Antioxidants protect body cells from free
radicals, which are not stable molecules that may result in harmful reactions
in the body, thereby causing disease. According to Shori
(2015), and Savoia
(2012), there are numerous
varieties of date palm fruit. The botanical name of the date palm, Phoenix
dactylifera L., is presumably derived from a Phoenician name
"phoenix", which means date palm, and "dactylifera" derived
from a Greek word "daktulos" meaning a finger Abreu
et al. (2012). The
"dactylifera" originates from the Hebrew word "dachel"
which describes the fruit's shape. Infectious diseases are caused by pathogenic
microorganisms, such as bacteria, viruses, parasites or fungi. Diseases can
spread, directly or indirectly, from one person to another. Infectious diseases
is next to leading cause of death world Cassir
et al. (2014).
Various plants usually serve as herbal medicine for the
treatment of infectious diseases. Plants vary with the respect to their potency
for healing diseases and their specificity as antimicrobial agents can be
ascertained Odeyemi
et al. (2017). Human populations are
affected by bacterial and fungal infections due to uncontrolled growth and
improper food habits and also there is increase in immune compromised
Agyare
et al. (2013). Antibiotics
are medicines used to prevent or cure microbial infections. Antibiotics have proven to be powerful
drugs for the control of infectious diseases and remain one of the most
discoveries in modern medicine Hussah
(2019). Their extensive and indiscriminate use
has, however, imposed a selective pressure upon bacteria, leading to the
emergence of antimicrobial resistance.
2. MATERIALS AND METHODS
2.1. Study Area
The fruit of Phoenix dactylifera was obtained from Lafenwa market
in Abeokuta, Ogun State. Lafenwa is located in Abeokuta, which is the capital city of Ogun State,
Nigeria; an approximately 61km / 38m away from other regions.
Figure 1
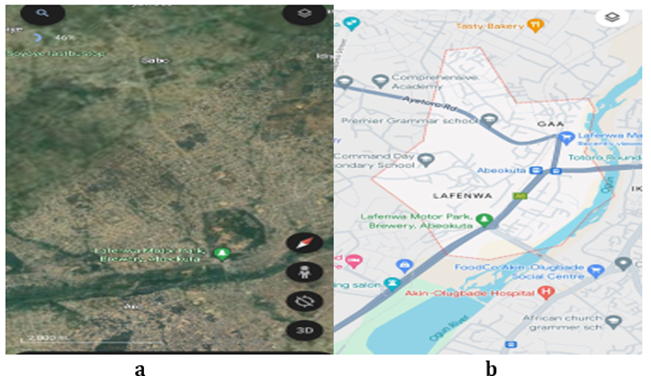
|
Figure 1 Study Area and Location Map |
2.2. Sample Preparation and Storage
The fruits of Phoenix dactylifera was air-dried for two weeks and pulverised into fine powder in a Marlex electroline 750 watts milling machine. The powder of each were kept in air tight container to retain its potency and avoid loss of odour.
2.3. Test Isolates
The test organisms used for this study were the clinical isolates collected from the Department of Medical Microbiology and Parasitology, Sacred Heart Hospital, Lantoro, Abeokuta. The isolates are Escherichia coli, Staphylococcus aureus, Bacillus cereus, Salmonella typhi and Aspergillus niger. The collected organisms on sterile agar slant and incubated at 37oC for twenty hours, which were even kept as stock culture on slant in the refrigerator set at 4oC.
2.4. Preparation
of Fruit Extracts
The fruit was rinsed, air dried and grinded into fine powder with an electric blender. Extraction of Phoenix dactylifera fruit extraction was done by soxhlet method. Ten gram (10g) each of dry powder of date fruit was added to the sample chamber of the soxhlet apparatus containing 100 mL of water, ethanol, and methanol. The extraction was done for 48 hours till the green colour of the plant materials disappeared after which the extract was collected and stored in airtight bottles and tested for antimicrobial activity.
2.5. Metabolite Constituents of Date Palm Fruits
Proximate
analyses were done on date palm fruit pulverised into powder to determine the
biochemical properties of the fruit and the effects on some microorganism and
ailment.
2.5.1.
Estimation of Crude Fibre
Five grams (5g) of sample was weighed on an analytical
balance and transferred to the volumentary flask. One hundred milliliter (100mL) of 1.25% Sulphuric acid
was measured and poured into the volumentary flask. The acid with the sample
was boiled under reflux for 45minutes. A sieve was used to trap the residue of
the boiled sample. The trapped residue was washed in quantifiable proportion of
hot water but allowed to drain. The residue above was transferred to the
volumentary flask and boiled again with 100millitre of 1.25% sodium hydroxide
solution (NaOH) for another 45 minutes.
A sieve was again used to trap the residue of the boiled sample. The
trapped residue was also washed in several portion of hot water and allowed to
drain. The residue was transferred into a weighed crucible where it was
transferred into an oven to obtain a constant weight at 105oC for 3
hours. The sample in the crucible was taken into the muffle furnace where it
was burnt. The ash left was weighed and the crude fibre was determined, thus;
![]() = W2-W3 x 100_
(1)
= W2-W3 x 100_
(1)
W1
|
W1=Weight
of the sample used |
|
W2= Weight
of the Crucible + sample after washing and
drying W3 = Weight
of crucible + ash |
2.5.2.
Determination of Fat Content
An empty beaker was weighed on analytical balance and noted.
One (1) gram of the sample was weighed into a separating funnel. Twenty (20)
milliliter of 96 % ethanol was added into the funnel and shake gently. Allow to
cool. Ten milliliter of Concentrated Sulphuric acid (H2SO4) was added. Twenty
(20) milliliter of petroleum ether was added to extract and shake well. For
emulsion to separate well,20 milliliter of ethanol and 20 milliliter of
petroleum ether was added for better extraction as many times as possible. The
separated fat extract was decanted. All the extracts were combined and
evaporate to dryness. The fat extract was weighed and calculated.
= W2-W1 x100 (2)
W3
|
W= Weight
of the sample used |
|
W1= Weight
of empty beaker |
|
W2= Weight
of empty beaker + fat after evaporation
(ADA, 2005) |
2.5.3.
Determination Protein Content
One (1) gram of the sample was weighed into a Kjeldahl
digestion flask. Fifteen gram (15g) of potassium sulphate and 0.5g of copper II
sulphate penthydrate were added. Thirty milliliter (30mL) of Concentrated
Sulphuric acid (H2SO4) was added. The sample was heated in the fume cupboard to
digest at 50oC until floating ceased. Then boiled at 80oC
until it is cleared. Two hundred (200) milliliter of distilled water and 25mL
of Sodium thiosulphate were added and mix. Anti-bumps were added and 50% of
110mL of NaOH was carefully added. The flask was connected to the distillation
apparatus and boiled at 80oC. One hundred and fifty (150) milliliter
of the distillate was collected. Five (5) drops of methyl red indicator was
added to the distillate and titrated with 0.1M of HCl.
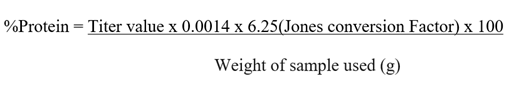
(ADA, 2005)
(3)
2.5.4.
Determination of Moisture Content
Two clean crucibles were dried in an oven and cooled in a
dessicator. The two cooled crucibles were weighed and recorded. One gram (1g)
of the sample was weighed in duplicate. The crucibles with its contents were
transferred into a hot air oven set at 105oC to dry for 3hours.
Using a pair of tongs, the crucibles were transferred into a dessicator,
allowed to cool, weighed and recorded.
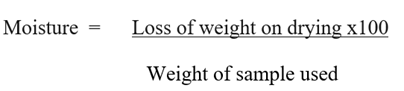
(ADA, 2005) (4)
2.5.5.
Determination of Ash Content
Two clean crucibles were dried in an oven and cooled in a
dessicator. One gram (1g) of the sample was weighed in duplicate into the
crucibles and recorded. The crucibles with its contents were transferred into a
muffle furnace set at 550oC until fully ashed for 5 hours. Using a
pair of tongs, the crucibles were transferred into a dessicator, allowed to
cool, weighed and recorded.
% Ash= Loss
of weight on ashing x100 (5)
Weight of sample used
![]() =
(W2-W3) X100
=
(W2-W3) X100
(W2-W1)
|
Weight of
the empty crucible W1 |
|
Weight of
crucible + sample before drying W2 |
|
Weight of
crucible + sample after drying W3 |
|
Weight of
sample taken W2-W1 |
|
Loss of
weight on ashing W2-W3
ADA (2005) |
2.5.6.
Determination of Carbohydrate
Content
The carbonhydrate content of the sample was determined using this formula:
Total Carbonhydrate= 100-(Weight of Crude fiber +Weight of protein+ Weight of ash+ Weight of moisture+ moisture content Weight of total fat). (6)
Where 100= Total weight of the sample. ADA (2005)
2.6. Determination of
antimicrobial activity Date Palm Fruit
Extract
Phoenix dactylifera (date palm fruit) was examined through agar well diffusion method. The adjustment of microbial cultures was made at 0.5 McFarland turbidity standards and innoculated on Mueller Hinton agar (MHA, oxoid) plate (diameter 9cm) in bacteria and Potato Dextrose agar (PDA) for fungi. The plate was flooded with 1ml of each of the standardized test organism, swirled and excess inoculum was carefully decanted. A sterile cork borer was used to make wells (6mm in diameter) on the agar plates. The extract was reconstituted in 50% dimethyl sulfoxide to give a concentration of 200 mg/ml. The culture plate was inoculated with the test microorganism. Each well was labeled appropriately. Control experiments were also carried out where the holes were filled with 200 mg of Ciprofloxacin (bacteria) and Fluconazole (Fungi) as positive controls. However, each extract was tested in triplicates. These were later left on the bench for one hour to give room to diffusion of the extracts. Thereafter, the plates were incubated at 37 0C and 280C for 48 hours for bacteria and fungi respectively. Measurement of the zone of inhibition around each of the wells determined antimicrobial activity for the extract.
2.7. Statistical analysis
The experiments with samples were performed in three segments and, where applicable; statistical analysis of the data obtained was done using one-way analysis of variance (ANOVA), and the difference between samples were determined by Duncan's multiple range test. The data were expressed through mean which is the product of standard deviation and values, being significantly considered at P < 0.01.
3. RESULTS AND DISCUSSION
Table 1 shows the proximate analysis of the date palm fruit that include the moisture content, fat content, ash content, crude fibre, crude protein, carbohydrate. Date palm fruit Phoenix dactylifera is high in carbohydrate content with 84.6%, which is source of energy followed by crude fibre that has 8.66%. It has 2.48% crude fibre, 2.27% ash content, 1.78% moisture content, 0.21% fat content.
Table 1
|
Table 1 Proximate Analysis (%) of
Dates Palm Fruit |
|
|
Parameters |
Proximate
(%) |
|
Moisture |
1.78 |
|
Fat |
0.21 |
|
Ash |
2.27 |
|
Crude
Fibre |
8.66 |
|
Crude
Protein |
2.48 |
|
Carbohydrate |
84.6 |
Antimicrobial
Activities of Date Palm Fruit Extract by Agar Well Diffusion Test.
Table 2 shows the
antimicrobial susceptibility of the
test organisms, Staphylococcus aureus, Escherichia coli, Salmonella typhi,
Bacillus cereus and Aspergillus niger to the aqueous, ethanoic
extract and control. All the above-mentioned microorganisms were susceptible to
the above-mentioned extracts. The individual reactions of the test organisms
against each solvent were discussed with their respective mean zone diameter. Table 2, also reveals the
inhibition mean zone of the growth of the microorganism to the extracts.
Ethanoic in date palm fruit have the highest antibacterial activity against Staphylococcus aureus having the mean
zone diameter of 28mm followed by Aqueous 21mm and compared with the control of
29mm. Ethanoic in date palm fruit have
the highest antibacterial activity against Escherichia
coli having the mean zone diameter of 29mm followed by the Aqueous of 19mm
compared with the control of 28mm. Ethanoic in date palm fruit have the highest antibacterial activity
against Salmonella typhi having the
mean zone diameter of 22mm followed by the Aqueous which is 16mm compared with
control of 29mm. Ethanoic in date palm fruit have the highest antibacterial
activity against Bacillus cereus
having the mean zone diameter of 22mm followed by the Aqueous which is 17mm
compared with control of 27mm. Ethanoic
in date palm fruit have the highest antibacterial activity against Aspergillus niger having the mean zone
diameter of 29mm followed by the Aqueous which is 22mm compared with the
control of 28mm.
Table 2
|
Table 2 Antibacterial Activity
of Date Palm Fruit Extract (Phoenix dactylifera) against some pathogen (mm) |
|||
|
Antimicrobial
activity of the extract (mm) |
Control |
||
|
Organisms |
Aqueous |
Ethanolic |
Antibiotics |
|
1. S.
aureus |
21.00 |
28.00 |
29.00 |
|
2. E.
coli |
19.00 |
29.00 |
28.00 |
|
3. S.
typhi |
16.00 |
22.00 |
29.00 |
|
4. B.
cereus |
17.00 |
22.00 |
27.00 |
|
5. A.
niger |
22.00 |
29.00 |
27.00 |
|
Control (Antibiotics for Bacteria-Ciprofloxacin,
Fungi- Fluconazole) |
|||
Minimum Inhibitory Concentration of the Date
Palm Fruit (Phoenix dactylifera) Extract
Table 3 below shows the results of the Minimum
inhibitory concentration of the date palm fruit extract against the test
organism and antimicrobial susceptibility of the test organisms, Staphylococcus aureus, Escherichia coli,
Salmonella typhi, Bacillus cereus and Aspergillus niger with aqueous,
ethanoic solvent. Result of this study showed that all the microorganisms were
susceptible to the above mention extract. Aqueous of date palm fruit extract
showed the highest MIC value for Staphylococcus
aureus having the value of 13mg/mL followed by ethanoic 5mg/mL compared
with 5mg/mL obtained from the control at the same concentration. Aqueous of
date palm fruit extract showed the highest MIC value for Escherichia coli
having the value of 19mg/mL followed by ethanoic 3mg/mL compared with 3mg/mL
obtained from the control at the same concentration. Aqueous extract of date
palm fruit extract showed the highest MIC value for Salmonella typhi having the value of 25mg/mL followed by ethanoic
19mg/mL compared with 2mg/mL obtained from the control at the same
concentration. Aqueous of date palm
fruit extract showed the highest MIC value for Bacillus cereus having the value of 25mg/mL followed by ethanoic of
the point 9mg/mL compared with 5mg/mL obtained from the Control at the same
concentration. Aqueous of date palm fruit extract showed the
MIC value for Aspergillus niger the
value of 6mg/mL followed by ethanoic 3mg/mL compared with 3mg/mL obtained from the control at the
same concentration. Minimum
Inhibitory Concentration (MIC) refers
to the minimum concentration for antimicrobial drug that impedes visible growth
of microorganism after overnight incubation with media. The small MIC value indicates that less fruit extract
(antimicrobial drug) is necessary for inhibiting growth of the organism,
therefore, the fruit extract (antimicrobial drug) with lower MIC value are more
effective.
Table 3
|
Table 3 Minimum Inhibitory Concentration Activity of
Extracts of Date Palm Fruit (mg/mL) |
|||
|
Extracts |
|
Control |
|
|
Organisms |
Aqueous |
Ethanolic |
Antibiotics |
|
S. aureus |
13.00 |
5.00 |
5.00 |
|
E. coli |
19.00 |
3.00 |
3.00 |
|
S. typhi |
25.00 |
19.00 |
2.00 |
|
B. cereus |
25.00 |
9.00 |
5.00 |
|
A. niger |
6.00 |
3.00 |
3.00 |
|
Control (Antibiotics for Bacteria-Ciprofloxacin,
Fungi- Fluconazole). |
|||
Minimum
Bactericidal/Fungicidal Concentration Activity of Extracts of Date Palm Fruit
Table 4 below shows result of the minimum
bactericidal/fungicidal concentration (MBC/MFC) of the test organism. Staphylococcus aureus, Escherichia coli,
Salmonella typhi, Bacillus cereus and
Aspergillus niger with aqueous, ethanoic solvent. Result of this study
showed that all the microorganisms were susceptible to the above mention
extract. Aqueous of date palm fruit extract showed the highest MBC value
for the Staphylococcus aureus with
value of 19mg/mL followed by ethanoic 13mg/mL compared with 6mg/ml obtained from
the control at the same concentration. Aqueous of date palm fruit extract
showed the highest MBC value for the Escherichia
coli with the value of 13mg/mL followed by ethanoic 5mg/mL compared with
3mg/ml obtained from the Control at the same concentration. Aqueous of date
palm fruit extract showed the highest MBC value for the Salmonella typhi with the value of 25mg/mL followed by ethanoic
19mg/mL compared with 2mg/mL obtained from the control at the same
concentration. Aqueous of date palm fruit extract showed the highest MBC value
for Bacillus cereus with the value of
25mg/mL followed by the ethanoic 9mg/mL compared with 6mm/mg obtained from the
control at the same concentration. Aqueous of date palm fruit extract showed
the highest MBC value for the Aspergillus
niger with the value of 13mg/mL followed by the ethanoic with 9mg/mL
compared with 5mg/mL obtained from the control at the same concentration.
Table 4
|
Table 4 Minimum Bactericidal Concentration of the
Extracts of Date Palm Fruit (mg/mL) Against Some Test Microorganism. |
|||
|
Extracts |
|
Control |
|
|
Test Organisms |
Aqueous |
Ethanolic |
Antibiotics |
|
S. aureus |
19.00 |
13.00 |
6.00 |
|
E. coli |
13.00 |
5.00 |
3.00 |
|
S. typhi |
25.00 |
19.00 |
2.00 |
|
B. cereus |
25.00 |
9.00 |
6.00 |
|
A. niger |
13.00 |
9.00 |
5.00 |
|
Control (Antibiotics for Bacteria-Ciprofloxacin,
Fungi- Fluconazole). |
|||
4. CONCLUSION
In this comprehensive study of the antimicrobial activities of date palm extracts, we have uncovered compelling evidence of their potent inhibitory effects against a range of test organisms, including Staphylococcus aureus, Escherichia coli, Salmonella typhi, Bacillus cereus, and Aspergillus niger. Both aqueous and ethanoic extracts of date palm fruit exhibited significant antimicrobial susceptibility, demonstrating their potential as natural agents for combating microbial infections. Ethanoic extracts, in particular, stood out with their remarkable antibacterial activity against Staphylococcus aureus and Escherichia coli, showcasing mean zone diameters of 28mm and 29mm, respectively. This performance surpassed that of the control, underlining the efficacy of date palm fruit extracts in inhibiting bacterial growth. Moreover, the Minimum Inhibitory Concentration (MIC) values further emphasized the superiority of ethanoic extracts, as they exhibited notably lower values compared to the control. These lower MIC values imply that ethanoic extracts require less fruit extract as an antimicrobial agent to hinder microbial growth, suggesting their potential as potent and cost-effective natural alternatives for combating bacterial and fungal infections. The findings provide valuable insights into the antimicrobial potential of date palm extracts, paving the way for further research and potential applications in the development of novel antimicrobial agents and therapeutics.
CONFLICT OF INTERESTS
None.
ACKNOWLEDGMENTS
None.
REFERENCES
Abreu, A. C., McBain, A. J., & Simões, M. (2012). Plants as
Sources of New Antimicrobials and Resistance-Modifying Agents. Nature Product
Reports, 29, 7–21.
Agyare, C., Dwobeng, A.S., Agyepong, N., Boakye, Y.D.,
Mensah, K.B., & Ayande, P.G. (2013).
Antimicrobial, Antioxidant, and Wound Healing Properties of Kigelia Africana
(Lam.) Beneth. and Strophanthus hispidus DC Advanced Pharmacol Science, 10.
American Diabetes Association (2007). Diagnosis and Classification of Diabetes Mellitus Care, 30, 42-46.
Cassir, N., Rolain, J.M., & Brouqui, P. (2014). ‘A New Strategy to Fight Antimicrobial Resistance: The Revival of Old
Antibiotics’. Frontiers in Microbiology, 5, 551.
Grover, N., & Patni, V. (2013). Phytochemicals Using Various Solvent Extracts and GC-MS Analysis of
Methanolic Extract of Woodfoodia Fruticosa (L) Kurz. Leaves. International
Journal of Pharmacology Science, 5, 291-295.
Hussah, A. A. (2019). Date
Palm (Phoenix Dactylifera L.) Fruit as Potential Antioxidant and Antimicrobial
Agent. Journal of Pharmacy and Bioallied Sciences.
Odeyemi, S., Afolayan, A., & Bradley, G. (2017). Phytochemical Analysis and Anti-Oxidant Activities of Albuca
Bracteata Jacq. and Albuca Setosa Jacq Bulb Extracts Used for the Management of
Diabetes in the Eastern Cape, South Africa. Asian Pac Journal of Tropical
Biomedical, 7(6), 577-584.
Patel, D.K., Prasad, S.K., Kumar, R., & Hemalatha, S.
(2012). An Overview on Antidiabetic Medicinal
Plants Having Insulin Mimetics Property. Asian Pacific Journal of Tropical
Biomedicine, 2, 320-330.
Rahimi, M. (2015). Anti-Diabetic Medicinal Plants used for
Diabetes Mellitus Bull. Env. Pharmacol Life Sciences, 4, 163-180.
Savoia, D. (2012).
Plant-Derived Antimicrobial Compounds: Alternatives to Antibiotics. Future
Microbiology, 7(8), 979–990.
Shori, A. B. (2015). Screening
of Antidiabetic and Antioxidant Activities of Medicinal Plants. Journal of
Internal Medicine, 13, 297-305.
Upadhyay, A., Upadhyaya, I., Kollanoor-Johny, A., & Venkitanarayanan, K. (2014). Combating Pathogenic Microorganisms using Plant-Derived Antimicrobials: A Minire View of the Mechanistic Basis. Biomed Research International, 24-18.
|
|
 This work is licensed under a: Creative Commons Attribution 4.0 International License
This work is licensed under a: Creative Commons Attribution 4.0 International License
© ShodhAI 2024. All Rights Reserved.